Water
Thinking about Water
If you were asked to name the different ways in which you depend on waterglossary term (opens in a new window), what would come to mind? You would probably say that you depend on water for drinking and staying hydrated, as well as for bathing, watering plants, and washing clothes. It soon becomes clear that water is necessary for many reasons. If you trace the history of water back billions of years, it is likely that water provided the liquidglossary term (opens in a new window) environment for life to begin. It is also likely that Earth’s first organisms, such as plants and animals, were aquatic. What are some other ways that water continues to sustain life today? What kinds of species live under, or depend on proximity to, water?
Oceans and large bodies of water are important for contributing to the water cycle, a process that impacts our climate and helps maintain a balance of thermal energy. The properties of water also make it possible for water to take on a solid form, known as ice. Water is one of the few substances that is less dense in its solid state than its liquid state. This explains why ice floats instead of sinks. Have you ever wondered what would happen if this property of water did not exist? What kind of organisms would be impacted if ice sank? How would this affect our polar regions where large sheets of ice and glaciers are found?
Explain Question
In what ways do the properties of water affect life on Earth?
Teacher Note
Use student responses to this formative assessment to evaluate their basic knowledge of the water cycle. Suggested use of this item includes students working in think-pair-shares to discuss their thoughts about the water cycle.
Before You Begin
What do I already know about water?
Teacher Note
This formative assessment is intended to address students’ prior knowledge of water’s chemical bonds and chemical properties. A suggested use of the item involves a whole-class or small-group discussion in which each statement is discussed by students to help formulate their answers.
Teacher Note
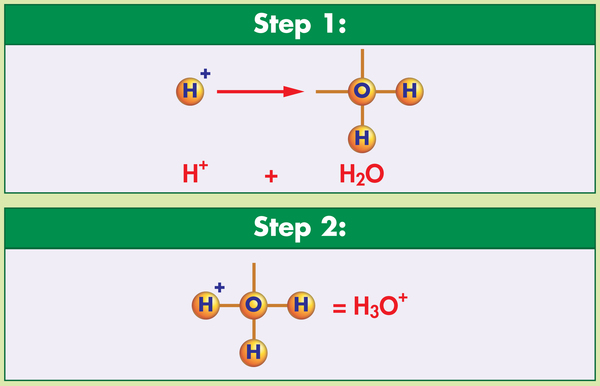
This formative assessment is intended to address student misconceptions about the properties of water. Some students may believe that water molecules are stable and that liquid water molecules remain in their H-O-H configuration. In fact, water is usually found in ionic form: hydroxide and hydronium ions. A suggested use of the item involves a whole-class or small-group discussion in which each statement is discussed by students to help formulate their answers.
Teacher Note
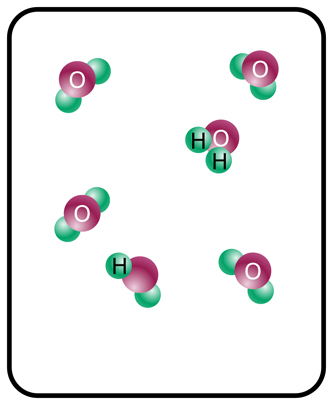
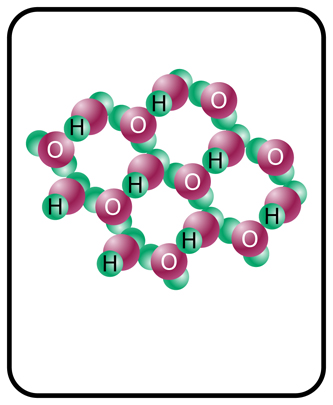
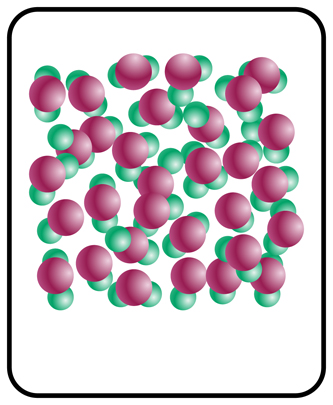
This formative assessment is intended to address students’ prior knowledge of the states of water and how the molecular structure changes depending on the state of matter. The activity can be performed in think-pair-shares.
- Solid
- Liquid
- Gas
Find out More About...
- understanding the basic atomic structure
- understanding how atoms form molecules
- distinguishing between different chemical bonds, including ionic, polar covalent, and nonpolar covalent bonds
Lesson Question
- What are the properties of water?